The US Centers for Disease Control and Prevention (CDC) have reported three deaths, eight cases of vision loss, and four cases of eyeball removal possibly linked to an over-the-counter eye drop made by a pharmaceutical company in Chennai. However, control samples taken during an inspection by India’s drug regulator showed that the quality was standard, with no bacterial contamination detected.
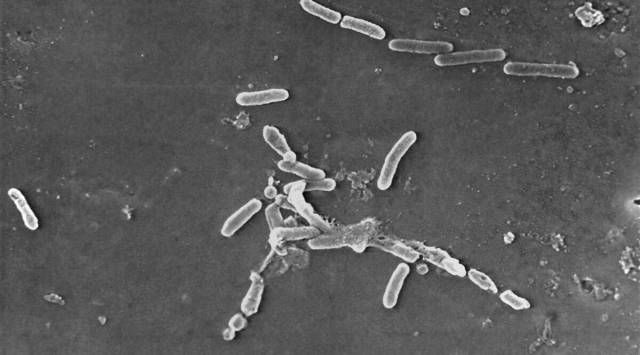
The infection, caused by the bacterial strain pseudomonas aeruginosa, which is resistant to the antibiotic carbapenem, was previously unseen in the US. The CDC is currently testing opened bottles for contamination and is awaiting results on unopened bottles.
The company, Global Pharma Healthcare, voluntarily recalled the product in February after the incidents were reported. Manufacturing of all eye care products at the company has been suspended as a precaution until investigations are completed.
Indian authorities have not received any official communication from the US, and have requested details from them. The manufacturing halt will continue until further notice.

































+ There are no comments
Add yours